Abstract
Background: Prospective real-life data is limited on the safety and effectiveness of rituximab biosimilars in Indian patients with non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL). This real-world study evaluated the safety and efficacy outcomes of rituximab biosimilar (Mabtas) plus chemotherapy (R-chemo) in Indian patients with B-cell NHL or CLL.
Methods: A prospective, observational, multicenter, single-arm, study of treatment-naïve B-cell NHL or CLL patients who were treated with first-line R-chemo treatment at 14 centers in India between February 2016 and November 2021 was conducted. This report provides an interim data analysis of this registry. The effectiveness endpoints included overall response rate (ORR, complete response [CR] + partial response [PR]), disease control rate (DCR, CR+PR+stable disease [SD]) progression-free survival (PFS) and overall survival (OS). Safety endpoints were adverse events (AEs), serious AEs, drug-related AEs, and AEs of special interest.
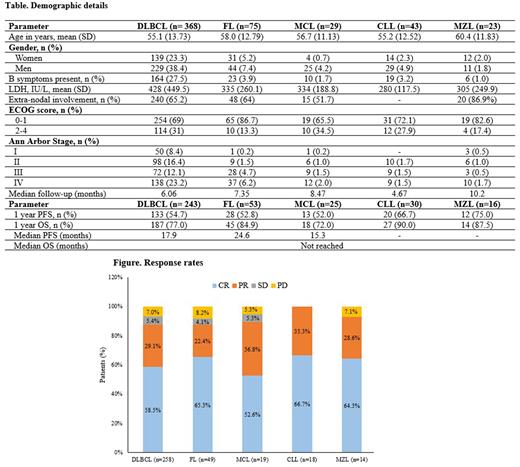
Results: A total of 596 treatment naïve CD20-positive patients were included in this interim analysis. Diffuse large B cell lymphoma (DLBCL, n=368) was the most common diagnosis, followed by follicular lymphoma (FL, n=75), CLL (n=43), mantle cell lymphoma (MCL, n=29) and marginal zone lymphoma (MZL, n=23) (Table); other lymphoma subtypes were reported in 58 patients. The ORR was 87.6% for DLBCL, 87.8% for FL, 100% for CLL, 89.5% for MCL and 92.9% for MZL; the DCR was 93%, 91.8%, 100%, 94.7%, and 91.9%, respectively (Figure). For DLBCL, FL, CLL, MCL and MZL, the one-year PFS rates were 54.7%, 52.8%, 52%, 66.7%, and 75%, respectively; the one-year OS rates were 77%, 84.9%, 72%, 90%, and 87.5%, respectively. The median PFS was 17.9, 24.6 and 15.3 months for DLBCL, FL and MCL; for CLL and MZL, the median PFS was not reached. The median OS was not reached for any indication in this study. Overall, R-chemo was well-tolerated.
Conclusions: Rituximab biosimilar based chemotherapy was effective and safe in real-world clinical practice as the first-line treatment for B-cell NHL and CLL.
Disclosures
Patel:Lambda Therapeutic Research Limited: Current Employment. Sharma:Intas Pharamceuticals Limited: Current Employment. Rajani:Intas Pharamceuticals Limited: Current Employment. Bunger:Intas Pharamceuticals Limited: Current Employment. Chaturvedi:Intas Pharmaceuticals Limited: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal